Resource | Mollie's Blog No 15
Meet the Scientists
As I head into the final 3 months of my time at MRC PPU, I am working towards setting up the two largest, longest and most complex experiments I will have completed in my project. I am both extremely excited and itching to get started but I cannot deny the underlying nerves I am also feeling. The results of these experiments could be very informative and provide new insights into the PINK1/Parkin pathway so I am determined to see these through to completion before I finish.
You may recall that some phosphatases (the class of proteins that act to remove phosphate tags) are only functional when their catalytic subunit (such as PP1 or PP2A) associates with one or more regulatory subunits to form a complex. The catalytic subunit is the part of the complex that performs the ‘chopping off’ function and the regulatory subunits act as guides to direct the catalytic subunit to a particular location within the cell and ensure that they only act on a very specific set of substrates.
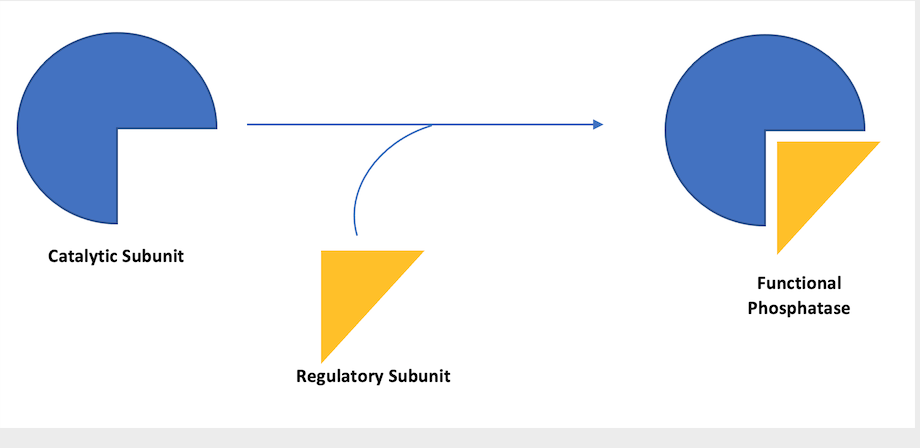
My final experiments are designed to try to uncover which regulatory subunit (or subunits) are required to associate with the PP1 catalytic subunit in order to direct it’s activity to phospho-Ubiquitin and cause the dephosphorylation I have observed in my past experiments. This is a very critical piece of the puzzle as, without their regulatory subunits, the catalytic subunits can be quite promiscuous.
In brief, I will systematically knockdown each of the known regulatory subunits and look to see if there is any affect on the levels of phospho-Ubiquitin. A gene knockdown is a technique frequently used in molecular biology that dramatically reduces the level of a specific protein in a cell. Any role that the targeted protein may have is lost once it is knocked down and this loss can be measured. In my experiment, I will be looking for a regulatory subunit that, when knocked down, results in an elevation in the levels of phospho-ubiquitin. This elevation in the levels of phospho-Ubiquitin imply that the phosphatase activity against it has been lost, in other words, without that specific regulatory subunit to guide it, the PP1 catalytic subunit can no longer perform its task of removing the phosphate tags.
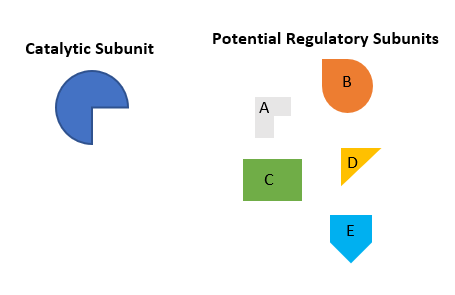
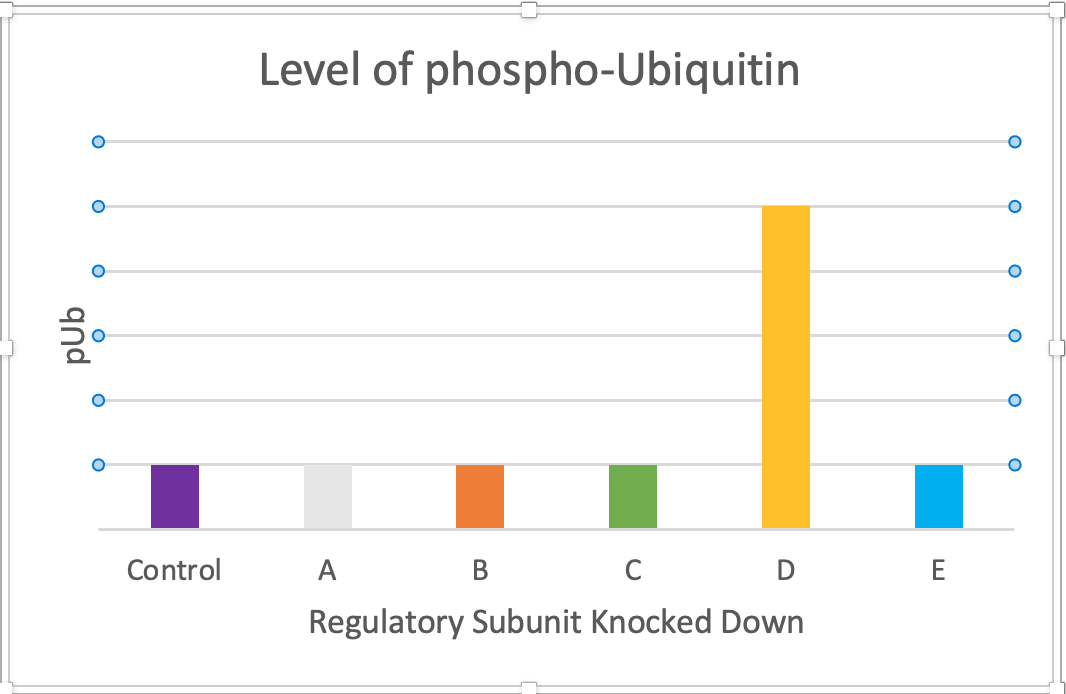
Above is a simplified example of the experiment – here there are 5 possible regulatory subunits which can all bind to the catalytic subunit, however, they may each be responsible for guiding the catalytic subunit to different areas in the cell and to different substrates. As I am looking for the subunit that guides the catalytic subunit to phospho-Ubiquitin, the level of phospho-Ubiquitin is used as the readout of activity. When proteins A, B, C and E are knocked down, there is no change to the level of phospho-Ubiquitin compared to the control. However, when D is knocked down, there is a significant increase in the level of phospho-Ubiquitin, indicating that subunit D must be crucial for directing catalytic activity to phospho-Ubiquitin.
In my actual experiment, I will be testing over 80 different regulatory subunits, a process that will take me several weeks. It is possible that none of these subunits will have any effect at all, or it may be that several seem to be involved, hence you may understand my butterflies about starting the experiment. Nonetheless, I feel well prepared to take on this big experiment and have all my fingers crossed that I will be able to finish my placement having at least whittled down the list of potential regulatory subunits. I look forward to keeping you up to date with (hopefully) lots of exciting news over the next 3 months.
