Resource | Mollie's Blog No 7
Meet the Scientists
A busy 2 weeks for me setting up the first experiments for my main project, as well as presenting at two lab meetings.
I have hit the ground running with my project in order to get some of the preliminary investigative experiments completed prior to Christmas. Two of the main experiments I have completed are known as time course and washout experiments. The former refers to a procedure that involves treating my cells for varying lengths of time with antimycin and oligomycin, compounds which artificially initiate the PINK1/Parkin pathway, as I explained in my 5th blog. I am then able to monitor the changes of specific proteins in the cell in response to this treatment. Essentially, I am looking to find the shortest treatment time possible at which I can detect sufficient changes in these proteins to visualise and quantify them. After I have determined a treatment time that yields a sufficient protein change, I conduct a washout experiment. This involves treating the cells with antimycin/oligomycin for the time I have determined in the time course experiment, after which I wash away the treatment and let the cells recover for different lengths of time. During this recovery time, I monitor further changes to the proteins I have been following and see if they return to the pre-treatment levels.
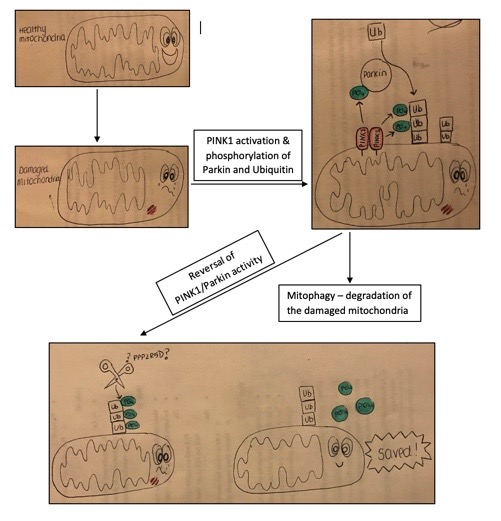
The purpose of these experiments is to show that there is a mechanism in the cells that is able to reverse the events triggered by the initiation of the PINK1/Parkin pathway in response to mitochondrial damage. Upon damage to the mitochondria, PINK1 (commonly mutated in Parkinson’s) becomes fixed to the outer mitochondrial membrane. Here, PINK1 activates several substrates including Parkin (another commonly mutated protein in PD) and a very small protein known as ubiquitin. PINK1 does this by adding phosphate groups (PO42-) to these substrates. Ubiquitin acts as a small tag that is fixed to the mitochondria by Parkin. Here, it signals to the cell that the mitochondria is damaged and should be destroyed. PPP2R5D, the protein I am studying, is predicted to be involved in the reversal of this signalling pathway and may act to remove these phosphates that PINK1 adds onto Parkin and Ubiquitin. In this way, PPP2R5D may be involved in a mechanism within the cell to prevent too many mitochondria being destroyed.
After a couple of attempts I have managed to gain some clear results for both the time course and washout experiments in two different types of cell. Following treatment with antimycin and oligomycin I observed a dramatic increase in the amount of phosphorylated ubiquitin within the cell, but after washing away the treatment, this phosphorylated ubiquitin disappeared with time. This suggests that there must be a mechanism in place that removes the phosphorylation. My next challenge is to investigate whether this mechanism requires PPP2R5D.
Each Friday we have a collaborative lab meeting with two of the other labs within MRC PPU in which researchers take it in turns to present their work, last week it was my turn. It would be fair to say that I was quite nervous about the prospect of showing my work to highly accomplished scientists but it was an extremely satisfying experience. Not only did I get to practice my presentation skills, but it also help me to underpin all the knowledge I have gained by having to explain it to others. The experience was also made very relaxed for the fact that I was able to do the whole thing from my home with a cup of tea in hand… one of the benefits of the new Zoom age!
With just one week left in the lab now before the holidays this will probably be my last blog before Christmas so I would like to take this opportunity to wish everyone a very merry Christmas. I cannot wait to tell you about the progress of this project in the New Year.