Resource | Mollie's Blog No 5
Meet the Scientists
Progress!
Shortly after writing my last blog I managed to achieve a successful PhosTag gel with some interesting results. The results have raised several interesting questions that will be exciting to explore in the future. It was great to see how, as a scientist, by investigating one question you can end up with many more to answer that you hadn’t realised were questions needing to be answered. This is such a fun, problem-solving element of science: you can start on one path and before long you’ve come to a 3, 4 or 5 way crossroads, all of which have to be explored to get to your end destination.
After achieving these PhosTag results, I moved on to learn another technique known as Immunoprecipitation. This is a technique that exploits the innate ability of antibodies to recognise a single protein to ‘pull’ a specific protein of interest from a cell lysate (this is the mixture of all the proteins from a cell). In doing so, you are effectively concentrating your protein of interest, making visualising it in techniques such as western blots considerably easier.
Learning this technique is key for verifying the preliminary results I gained for my mini project a few weeks ago. Just as a brief reminder, in this project I am investigating the role of Parkin, a commonly mutated protein in Parkinson’s Disease, in the phosphorylation (the addition of a small chemical tag) of a group of proteins known as the Rabs. By ‘pulling out’ the specific Rab protein I am studying from the complex mixture of proteins that comprise the cell lysates, I will be drastically reducing any background/interference that may come from other proteins in the sample. This will allow me to produce a very clear result as to whether the Rab protein has been phosphorylated or not under a very particular set of conditions. Is this brief enough or can you simplify it a bit more as you have already explained it in a previous blog.
As I described in my third blog, the proteins that the lab is focused on are all elements of the PINK1/Parkin pathway, a mechanism by which cells remove damaged mitochondria (the powerhouse of the cell). In Parkinson’s Disease mutations can arise in the proteins of this pathway causing aberrant mitochondrial regulation. To study this pathway, we need to be able to damage the mitochondria so that the PINK1/Parkin clearance mechanism becomes activated. The mitochondria have a double membrane. Importantly under normal conditions there is a charge difference across the inner membrane with the outside being more positive and the inside more negative. This charge difference, referred to as a potential difference, is caused by the transport of electrons along the inner membrane through a series of protein complexes (represented by the cars in the diagram). 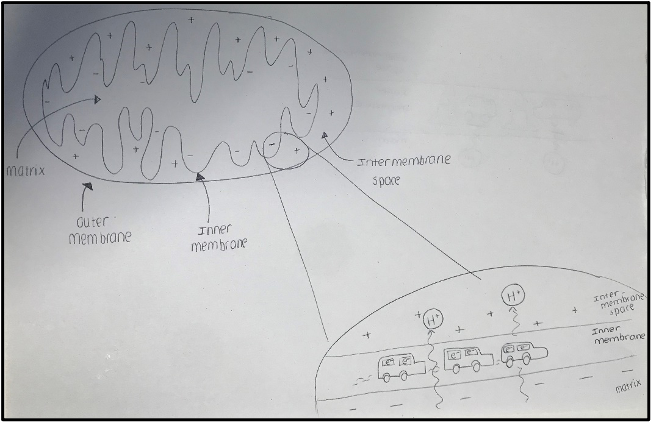 Concurrently, the complexes also transport positively charged Hydrogen ions from the matrix into the intermembrane space – much like a car releasing emissions. This process, known as oxidative phosphorylation is key to the generation of energy by the mitochondria. There are several substances that we can use to bring a stop to this continuous flow of traffic…. We can either directly interfere with the mechanics of the cars to stop them from working using two inhibitors (Antimycin and Oligomycin) or we can use another substance called CCCP to actively transport Hydrogen ions back into the matrix. Either of these methods results in a dissipation of the potential difference across the inner membrane resulting in a disruption to energy production. The mitochondria become unproductive and the PINK1/Parkin pathway is initiated to remove them.
Concurrently, the complexes also transport positively charged Hydrogen ions from the matrix into the intermembrane space – much like a car releasing emissions. This process, known as oxidative phosphorylation is key to the generation of energy by the mitochondria. There are several substances that we can use to bring a stop to this continuous flow of traffic…. We can either directly interfere with the mechanics of the cars to stop them from working using two inhibitors (Antimycin and Oligomycin) or we can use another substance called CCCP to actively transport Hydrogen ions back into the matrix. Either of these methods results in a dissipation of the potential difference across the inner membrane resulting in a disruption to energy production. The mitochondria become unproductive and the PINK1/Parkin pathway is initiated to remove them.
The last two weeks have been extremely satisfying as I feel as though I have gained several good quality results using all the techniques I have learnt so far: western-blotting, PhosTag gels and immunoprecipitation. I am also beginning to refine my experimental technique, becoming much more time effective and efficient, I hope this means I will be able to keep moving forward to my next mini project by the time I write my next blog.
